Potentially modifiable dementia risk factors in all Australians and within population groups: an analysis using cross-sectional survey data
September 2023
Summary
Background
Dementia is the second leading cause of disease burden in Australia. We aimed to calculate the population attributable fractions (PAFs) of dementia attributable to 11 of 12 previously identified potentially modifiable health and social risk factors (less education, hearing loss, hypertension, obesity, smoking, depression, social isolation, physical inactivity, diabetes, alcohol excess, air pollution, and traumatic brain injury), for Australians overall and three population groups (First Nations, and those of European and Asian ancestry).
Methods
We calculated the prevalence of dementia risk factors (excluding traumatic brain injury) and PAFs, adjusted for communality, from the cross-sectional National Aboriginal and Torres Strait Islander Health Survey (2018–19), National Aboriginal and Torres Strait Islander Social Survey (2014–15), National Health Survey (2017–18), and General Social Survey (2014) conducted by the Australian Bureau of Statistics. We conducted sensitivity analyses using proxy estimates for traumatic brain injury (12th known risk factor) for which national data were not available.
Findings
A large proportion (38·2%, 95% CI 37·2–39·2) of dementia in Australia was theoretically attributable to the 11 risk factors; 44·9% (43·1–46·7) for First Nations Australians, 36·4% (34·8–38·1) for European ancestry, and 33·6% (30·1–37·2) for Asian ancestry. Including traumatic brain injury increased the PAF to 40·6% (39·6–41·6) for all Australians. Physical inactivity (8·3%, 7·5–9·2), hearing loss (7·0%, 6·4–7·6), and obesity (6·6%, 6·0–7·3) accounted for approximately half of the total PAF estimates across Australia, and for all three population groups.
Interpretation
Our PAF estimates indicate a substantial proportion of dementia in Australia is potentially preventable, which is broadly consistent with global trends and results from other countries. The highest potential for dementia prevention was among First Nations Australians, reflecting the enduring effect of upstream social, political, environmental, and economic disadvantage, leading to greater life-course exposure to dementia risk factors. Although there were common dementia risk factors across different population groups, prevention strategies should be informed by community consultation and be culturally and linguistically appropriate.
Funding
Australian National Health and Medical Research Council and University College London Hospitals’ National Institute for Health Research (NIHR) Biomedical Research Centre, and North Thames NIHR Applied Research Collaboration.
Introduction
As the number of older adults (aged ≥65 years) increases,1 more people are projected to be living with dementia, resulting in rising social, health, and economic costs globally.2
Dementia is the second leading cause of disease burden and death overall in Australia,3 and a national health priority since 2012.4 Almost one in ten older Australians, or more than 400 000 people, currently live with dementia, with numbers expected to more than double by 2060.3 The economic cost is also projected to double to AU$36·8 billion.2
Risk factor modification is a leading approach to prevention, and many high-income countries have reported that despite more people living with dementia, there is a decrease in age-related incidence in people where risk factors have been ameliorated.1
The Lancet Commission on Dementia Prevention, Intervention, and Care 1 used population attributable fractions (PAFs), to estimate that 39·7% of dementia cases worldwide were attributable to 12 potentially modifiable risk factors: less education, hearing loss, hypertension, obesity, smoking, depression, social isolation, physical inactivity, diabetes, alcohol excess, traumatic brain injury, and air pollution. However, these risks vary between countries and within subpopulations, and estimates range from 39·5–55·8% in China, India, and Latin America 5 to 48·2% in Brazil.6
In Canada, dementia PAF was higher in the Indigenous than the non-Indigenous population.7 In New Zealand,8 the PAF for the four largest population groups (Europeans 47·6%, Māori 50·8%, Pacific Peoples 50·8%, and Asians 40·8%) differed from the overall national estimate (47·7%). In London, UK,9 identifying as an ethnic minority or having lower socioeconomic status was associated with more preventable dementia than known health risk factors. This highlights that both health risk factors and social determinants are important in addressing dementia prevention.
Research in context
Evidence before this study
The Lancet Commission on Dementia Prevention, Intervention, and Care found that approximately 40% of dementia worldwide was attributable to 12 potentially preventable risk factors (ie, less education, hearing loss, hypertension, obesity, smoking, depression, social isolation, physical inactivity, diabetes, alcohol excess, traumatic brain injury, and air pollution). In March, 2022, we systematically reviewed the literature on dementia prevention in Australia searching PubMed with the key words: “dementia”, “prevention”, “population attributable fractions (PAFs)”, “attributable fractions”, “ethnicity”, and “Australia” for studies in English. Two studies had investigated PAFs for dementia in Australia, one using seven risk factors, and one using 11 risk factors in First Nations residents of the Torres Strait and Northern Peninsula Area of Far North Queensland. We found no studies that had used PAFs to calculate the proportion of potentially preventable dementia across different population groups in the whole of Australia.
Added value of this study
We used Australian national survey data to calculate how much dementia burden in Australia might be attributable to 11 potentially modifiable health and social risk factors. In sensitivity analyses we calculated the contribution of traumatic brain injury (12th risk factor) to overall dementia risk. To our knowledge, this was the first study in Australia to explore PAF estimates across different population groups: First Nations Australians, Australians of European ancestry, and Australians of Asian ancestry.
Implications of all the available evidence
We found that 38% of dementia in Australia was due to 11 potentially preventable risk factors, which increased to 41% when traumatic brain injury was included. Physical inactivity, hearing loss, and obesity were the highest contributors for Australia overall and all population groups. Overall PAF estimates were higher in First Nations Australians, which probably reflects upstream social determinants and a history of disadvantage. These findings add to the growing knowledge on potentially preventable dementia and modifiable risk factors globally. They could guide culturally specific dementia risk reduction programmes at the population level and highlight the need to strengthen data on dementia risk across different population groups.
Australia is a diverse country in terms of population groups and cultures. A third of the population is born overseas, and the predominant ancestry groups are European (57·3%) and Asian (17·4%).
10 The First Nations peoples of Australia—Aboriginal and Torres Strait Islander people—are among the oldest continuous populations in the world and comprise 3·8% of Australia’s population.
11 The number of older people living with dementia is projected to increase for Australians from ethnic and migrant backgrounds 12 and First Nations Australians.13 To date, there is no estimate of how much dementia in Australia is due to the potentially modifiable risk factors identified by the Lancet Commission 1 and whether this varies across population groups. The only previous dementia PAF study of the entire Australian population used seven risk factors and estimated that half (48·4%) of dementia cases were potentially preventable.14 Similarly, although 11 risk factors accounted for a third (34·9%) of dementia cases in Torres Strait Islanders,15 this work only focused on one of Australia’s many First Nations peoples.
This study aimed to use publicly available national survey data to calculate PAFs for 11 potentially modifiable risk factors as identified by the Lancet Commission,1 for Australia and, for the first time, examine how this varied across three population groups (First Nations Australians, and Australians of European ancestry and Asian ancestry). National prevalence estimates for traumatic brain injury, the 12th risk factor, were unavailable; however, sensitivity analyses were conducted using proxy estimates for this risk.
Methods
Data sources for risk factor prevalence
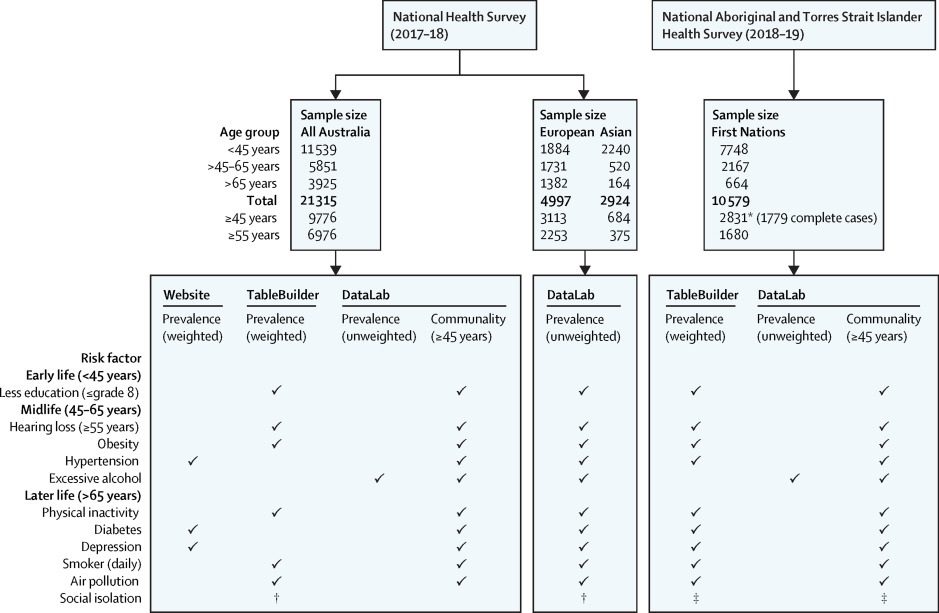
Data in this study were obtained from the Australian Bureau of Statistics (ABS). The ABS conducts routine national surveys using representative samples of the Australian population and then weights the survey responses to larger populations to improve the generalisability of findings. The current study used weighted data where possible, obtained from the ABS website, or their TableBuilder online platform (figure 1). Unweighted data, hosted on the ABS DataLab, were used for selected conditions and population groups where estimates were unavailable from the other sources. Data from the ABS surveys were used to create risk factors matching the Lancet Commission definitions and age groups for a life-course model (panel).1
Figure 1Data sources and samples sizes, by age groups and population groups, for dementia risk factor prevalence estimates and communality estimates, from the ABS
Panel 1
Definitions of potentially modifiable dementia risk factors from the Lancet Commission on Dementia Prevention, Intervention, and Care,
1 using self-reported survey data from the Australian Bureau of Statistics, National Aboriginal and Torres Strait Islander Health Survey (2018–19), the National Aboriginal and Torres Strait Islander Social Survey (2014–15), the National Health Survey (2017–18), and the General Social Survey (2014)
Obesity
BMI ≥30 kg/m2, based on height and weight measured at the time of survey response, or self-reported height and weight if no physical measurements were available.
Physical inactivity
Did not meet the Australian Department of Health 2014 Physical Activity and Sedentary Behaviour guidelines, based on self-report. For adults aged 18–64 years, physical activity guidelines recommend 150–300 min of moderate or 75–150 min of vigorous physical activity, or an equivalent combination of both, per week. For adults aged 65 years and older, the guidelines recommend at least 30 min of moderate intensity physical activity on most days of the week.
Smoker
Smoking any number of cigarettes daily.
Less education
8 years or less of formal school education completed.
Diabetes
Reported having diabetes as a long-term condition, which was defined as current and had lasted, or was expected to last, for 6 months or more.
Hypertension
Ever been told by a doctor or nurse that they had hypertension as a long-term condition.
Depression
Reported having depression or feelings of depression as a long-term condition.
Hearing impairment
Reported deafness, partial deafness, or hearing loss, as a long-term condition.
Excessive alcohol consumption
Drinking more than 21 units (168 g or 213 ml) of alcohol weekly.
Social isolation
Frequency of contact with family or friends outside the household was less than once per month.
Air pollution
Living in an urban area according to the Australian Statistical Geography Standard digital boundaries.
Survey responses were self-reported unless otherwise indicated. The interview questionnaires used by the Australian Bureau of Statistics to obtain the information in this panel are available from the National Health Survey, the General Social Survey, the National Aboriginal and Torres Strait Islander Health Survey, and the National Aboriginal and Torres Strait Islander Social Survey websites.
Risk factor prevalence for First Nations Australians was obtained from the 2018–19 National Aboriginal and Torres Strait Islander Health Survey (NATSIHS)16 and the 2014–15 National Aboriginal and Torres Strait Islander Social Survey (NATSISS).17 The NATSIHS collected health-related information for 10 579 First Nations people of all ages in non-remote and remote areas of Australia, although physical activity was not collected in remote areas.16
Survey responses were weighted to the 2018 First Nations estimated population. The NATSISS collected self-reported information across key areas of social interest for 11 178 First Nations Australians.17 Data from the NATSIHS were used for all risk factors except social isolation, which was sourced from the NATSISS. Risk factor prevalence for all Australians was obtained from the 2017–18 National Health Survey (NHS)18 and the General Social Survey (GSS).19 The NHS is repeated every 3 years to collect data on health conditions, risk factors, and demographic and socioeconomic information for the whole population. The 2017–18 NHS sampled 21 315 people and weighted results to Australia’s 2017 estimated resident population. The GSS is conducted every 4 years to collect information on the social characteristics, wellbeing, and social experiences of people in Australia aged 15 years and older. For the current study, data from the NHS were used for all risk factors except social isolation, which was sourced from the GSS.
The ABS did not categorise participants into European or Asian ancestry in the NHS and GSS; consequently, weighted estimates were unavailable for these two populations. Instead, unweighted prevalence estimates were derived using raw data in the ABS DataLab. First, ancestry was derived from parental country of birth using the broad groups classification in the ABS 2019 Australian Standard Classification of Cultural and Ethnic Groups.
20 The ABS developed this standard through consultation with academics, ethnic and community groups, and stakeholders involved in cultural diversity data. A dichotomous variable was created in DataLab to identify survey respondents of European ancestry, which was defined as having either parent from northwest Europe or southern and eastern Europe, or from both. We created an Asian ancestry dichotomous variable defined as having either parent from southeast Asia, northeast Asia, or southern and central Asia. Analyses of risk factor prevalence were limited to participants of the NHS who met these definitions of European and Asian ancestry. The categories were not mutually exclusive, as participants could have one parent from either region. As a result, some participants might have been classified as yes on both dichotomous ancestry variables and counted in both analyses. The definition of ancestry in this study was expected to encompass survey respondents who themselves migrated to Australia, and those who were born in Australia to parents who migrated. Ancestry was only derivable in the ABS DataLab, so all risk factor prevalence estimates for Australians of European or Asian ancestry were from the ABS DataLab. The exception was social isolation, which came from the GSS. In the GSS, participant country of birth was collected and used in this study as a proxy indicator of ancestry.
The Far North Queensland Human Research Ethics Committee (HREC) provided ethics approval for this study through an amendment (AM/2022/QCH/41243) to an existing project (HREC/18/QCH/92-1262), which examined dementia risk factors in one of Australia’s many First Nations populations.
Community support for these national PAF analyses was also recognised through an Australian National Health and Medical Research Council (NHMRC) Centres of Research Excellence (CRE) grant (Teaching, Research And Community Knowledges [On TRACK]: CRE promoting brain health with older Aboriginal and Torres Strait Islander peoples). Senior authors ES, DLG, ST, KR, and SR are investigators on this CRE. The CRE builds on the NHMRC Aboriginal and Torres Strait Islander Dementia Research and Translation Roadmap, which is based on community and stakeholder engagement, and highlighted risk factor detection and prevention as priorities for research. Further details on community engagement are available in appendix 1 (pp 1–2).
Outcomes
We calculated unadjusted PAFs using relative risk (RR) estimates for dementia and the prevalence of risk factors. We used the meta-analyses in the Lancet Commission 1 for RR estimates.
Since individuals can have several risk factors, and risk factors tend to cluster, we weighted PAF estimates for communality, which measures the variance in observed risks accounted for by common factors,1 and generated adjusted PAF estimates (appendix 1 pp 5–6, appendix 2). All analyses were performed using Stata 15 and Microsoft Excel version 2307.
Statistical analysis
Estimates were weighted for communality to derive individual and total adjusted PAFs. To obtain communality, we used unit record data from the ABS DataLab and performed estimates in two stages: preparation of unit record data and tetrachoric correlation matrix and principal components analysis.
In stage 1, ten dichotomous risk factors were derived for each survey respondent aged 45 years or older to match the risk factors on the ABS website and in TableBuilder. This process was performed for all Australians using NHS data and repeated for First Nations using NATSIHS data, so that communality estimates would be specific to these respective populations. Social isolation, the 11th risk factor, was in separate surveys (ie, the NATSISS and the GSS), and was not included in the calculation of communality for these two populations. Instead, we applied the average communality for the 10 risk factors to social isolation for each population, as in previous studies.1, 6, 8 Additionally, the communality estimates for all Australians were used for Australians of European or Asian ancestry.
In stage 2, the ten dichotomous risk factors were used to calculate communality for the NATSIHS and NHS separately (appendix 1 p 5). First, a tetrachoric correlation matrix of ten risk factors was created with Stata, command tetrachoric. Tetrachoric correlations use complete case analysis, so data for First Nations residents in remote areas were excluded as their physical activity levels were not recorded in the NATSIHS. The sample sizes for the tetrachoric correlations were 9776 for all Australians and 1779 for First Nations.
A principal components analysis was then run on the correlation matrixes using Stata generating components, eigenvalues, and component loadings. The component loadings used were normalised to their respective eigenvalue. The current study retained eigenvalues of 1 or greater, as this criterion has been used in similar studies and any component with an eigenvalue of 1 or greater explains more variance than a single observed variable. Communality was then calculated as the sum of the square of all component loadings (appendix 1 pp 5, 8–12).
Sensitivity analyses
The prevalence estimates of risk factors for NHS participants of European or Asian ancestry were not weighted to their respective Australian populations. Additionally, the number of participants of Asian ancestry with alcohol consumption and depression did not meet the ABS anonymity requirements for public release, and broader age groups, with more participants, were needed for these indicators (table). To account for these limitations, the main analyses were repeated as sensitivity analyses using prevalence estimates for participants aged 45 years or older for all population groups (appendix 1 pp 13–14).
TablePAFs for 11 potentially modifiable dementia risk factors in Australia, for all Australians, Australians of European and Asian ancestry, and First Nations Australians
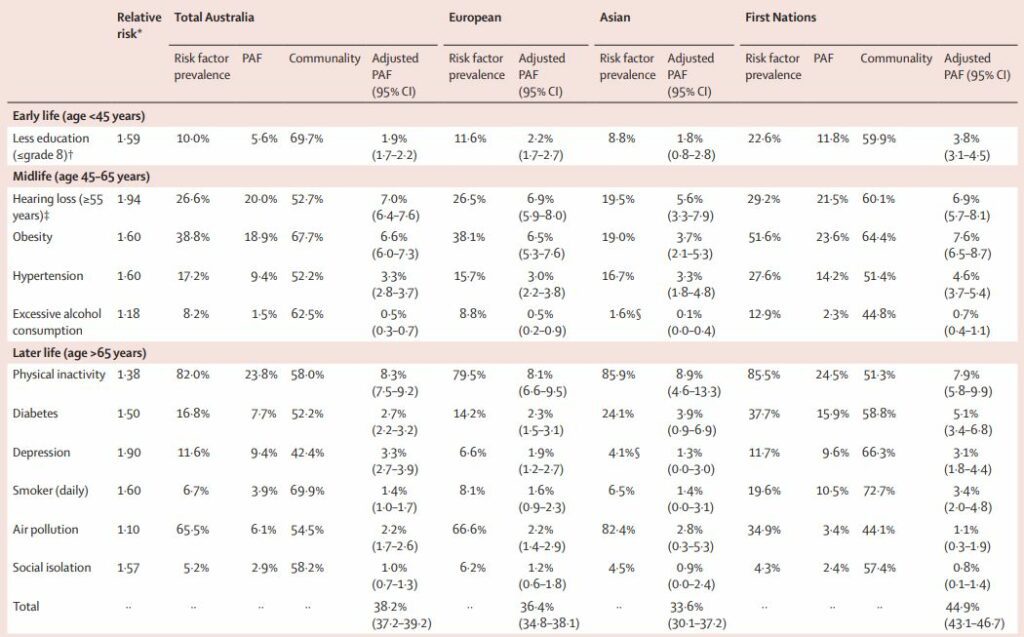
* Relative risk based on estimates from the Lancet Commission on Dementia Prevention, Intervention, and Care.1
† The prevalence of less education was estimated for people aged 45 years and older; however, the question about highest education achieved was presumed to relate to their early life. Grade 8 refers to the eighth year of formal school-based education in Australia.
‡ The prevalence of hearing impairment was estimated for people aged 55 years and older.
§ The prevalence of excessive alcohol consumption and depression were based on people aged 45 years and older for Asian ancestry. The CIs for PAF estimates were calculated using the standard method for 95% CIs for proportions, where the sample size was the number of participants in each of the national surveys, by age group. These data were obtained from the Australian Bureau of Statistics DataLab. The number of participants for the age group 45–65 years was: 5851 total Australia, 1731 European, 520 Asian, and 2167 First Nations; for the older than 65 years group it was 3925 total Australia, 1382 European, 164 Asian, and 664 First Nations; for the 45 years and older group it was 9776 total Australia, 3113 European, 684 Asian, and 2831 First Nations; and for the 55 years or older group it was: 6976 total Australia, 2253 European, 375 Asian, and 1680 First Nations. The sample size for the total CIs was based on participants aged 45 years and older. The formulas used for calculations are detailed in appendix 1 (p 6).
As there were no traumatic brain injury prevalence estimates for Australia, the main analyses were repeated using a traumatic brain injury prevalence of 12·1% (appendix 1 pp 15–16) from a meta-analysis of traumatic brain injury prevalence 21 as used by the Lancet Commission.1 For First Nations Australians, traumatic brain injury prevalence came from a dementia prevalence study of urban and regional Aboriginal Australians aged 60 years or older.22 The traumatic brain injury estimate in this study, 29·1%, fell between two estimates from other Australian studies (ie, 18% and 51%).15,23 The average communality of all other risks was used to weight PAF estimates for traumatic brain injury.
The main analyses were repeated using a broader definition of less education (ie, ≤10 years of formal education; appendix 1 pp 17–18). This definition was comparable to other similar PAF studies 14 and potentially more applicable to Australia than the Lancet Commission definition of no education after age 12–14 years.1 Appendix 1 (pp 19–20) summarises the source of prevalence data for all health indicators, by population group, and whether the prevalence estimates used in this study were weighted or unweighted. The CIs for PAF estimates were calculated using the standard method for 95% CIs for proportions, where the sample size was the number of participants in each of the national surveys, by age group. These data were obtained from the ABS DataLab.
Role of the funding source
The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
Results
The table shows prevalence estimates of potentially modifiable dementia risk factors, their communality, and unadjusted and adjusted PAFs for the different population groups. After adjusting for communality, 38·2% (95% CI 37·2–39·2) of dementia cases in the Australian population were theoretically attributable to 11 potentially modifiable risk factors. This proportion was 44·9% (43·1–46·7) for First Nations Australians, 36·4% (34·8–38·1) for Australians of European ancestry, and 33·6% (30·1–37·2) for Australians of Asian ancestry. Figure 2 shows the adjusted PAFs for Australia overall, and for the different population groups.
Figure 2Population attributable fractions, adjusted for communality, for 11 potentially modifiable dementia risk factors in Australia shown for all Australians, residents of European and Asian ancestry, and First Nations Australians
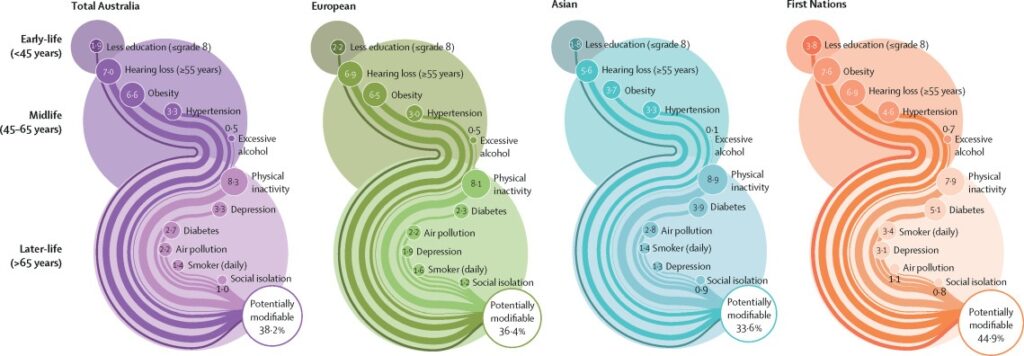
Physical inactivity had the highest adjusted PAF for all Australians (8·3%, 95% CI 7·5–9·2) and each population group (table). Hearing loss and obesity were the next major contributors for Australians overall, for First Nations Australians, and those of European ancestry. In contrast, excess alcohol and social isolation had the lowest contributing individual PAF estimates across all groups. There were some differences by population group for the remaining risk factors. First Nations had higher PAF for smoking (3·4%, 2·0–4·8) compared with all Australians (1·4%, 1·0–1·7). Additionally, the contribution of diabetes was greater in First Nations (5·1%, 3·4–6·8) than all Australians (2·7%, 2·2–3·2) and Europeans (2·3%, 1·5–3·1). The contribution of less education was greater in First Nations (3·8%, 3·1–4·5) than all Australians (1·9%, 1·7–2·2) and those of both European and Asian ancestry. For those of Asian ancestry, the contribution of obesity (3·7%, 2·1–5·3) was lower than for all Australians (6·6%, 6·0–7·3). Although there were differences in other risk factors between groups, these had overlapping CIs.
In sensitivity analyses using broader age groups (ie, ≥45 years) results were similar to those of the main analyses (appendix 1 pp 13–14). Although the total adjusted PAF increased to 40·1% (95% CI 39·2–41·1) for all Australians, the top three risks remained the same, as did the trends in total adjusted PAFs between population groups. However, the contribution of certain risks changed; hypertension and smoking increased, whereas diabetes decreased. Appendix 1 (pp 15–16) shows the adjusted PAF for all Australians increased to 40·6% (39·6–41·6) and for First Nations Australians to 49·5% (47·7–51·4), when estimates for traumatic brain injury were included. Similarly, when less education was defined as 10 years or fewer of formal education (appendix 1 pp 17–18), the weighted PAF for this risk became the highest for First Nations Australians (9·5%, 8·4–10·6) and the second highest for all Australians (7·0%, 6·5–7·5).
Discussion
We found that 38·2% (95% CI 37·2–39·2) of dementia in the Australian population was attributable to 11 potentially modifiable risk factors, with variation across three population groups (First Nations Australians, Australians of Asian ancestry, and Australians of European ancestry). The overall PAF estimate for First Nations peoples (44·9%, 43·1–46·7) was higher than estimates for people of European (36·4%, 34·8–38·1) and Asian (33·6%,) 30·1–37·2) ancestry. Physical inactivity, obesity, and hearing loss contributed to around half of this risk for all Australians, and all population groups. Excess alcohol and social isolation had the lowest PAF estimates across all groups. Although there was some variation in individual risks between different population groups, most differences had overlapping CIs.
The overall PAF estimate for 11 risk factors was similar to global results (39.7%),1 but lower than New Zealand (47·5%),8 Brazil (48·2%), 6 and previous Australian estimates (48·4%).14 At least one of obesity, physical inactivity, and hearing loss was in the top three risks for almost every country with similar analyses,
1, 5, 7, 8, 14, 15 suggesting a global pattern of dementia risk. They also align with New Zealand, where hearing loss and obesity were among the top three risks.8 Our overall PAF estimate from 11 risk factors was lower than a previous Australian study (48·4%) incorporating seven risks.14 However, the source of RRs, age groups for prevalence estimates, definitions of less education, and source of communality estimates all differed from our study. We found physical activity was more important for dementia prevention compared to the only other dementia PAF study with an Australian First Nations population.15 That study had limited measures of physical activity, which highlights the importance of using culturally appropriate and validated measures.
The higher rate of risk factors in First Nations communities is consistent with New Zealand,8 where dementia PAFs were higher in Māori and Pacific peoples than European and Asian populations, and Canada where modifiable risks for Alzheimer’s disease were higher in Indigenous than Non-Indigenous people.7 These patterns suggest that modifiable dementia risks across First Nations populations are influenced by similar cultural and historical factors, such as the enduring effect of colonialism, and shared ongoing experiences of trauma, violence, racism, marginalisation, and environmental and socioeconomic adversity. 24 These experiences contribute to the higher levels of preventable dementia risk factors, such as fewer years of education, and more smoking, 24, 25 alcohol use, diabetes,26 and traumatic brain injury. These trends are reflected in our findings, where the prevalence, and thus PAF estimates, of smoking and diabetes were greater in First Nations than all Australians, and low education had a greater impact compared to all other groups. Given that dementia develops at an earlier age in First Nations Australians, 22 our results suggest this trend reflects higher cumulative life course exposure to modifiable risks. A similar pattern exists among disadvantaged minority populations in other settings. In London, for example, lower socioeconomic status and belonging to an ethnic minority group were associated with increased risk of dementia independently of eight known risk factors;9 at the same time in the UK overall, people of an ethnic minority group develop dementia and die at a younger age than the majority of the population with dementia. 27
Our results highlight shared dementia intervention priorities across population groups. However, strategies must use culturally and linguistically tailored messaging and solutions for each intervention group, and tackle underlying causes, to achieve the same goal of risk factor reduction.28 For example, First Nations Australian children often have recurrent otitis media, leading to a younger age of hearing loss than other groups. Hearing loss prevention would need to address underlying factors such as poverty and improve early treatment, screening for complications, and culturally appropriate resources.29 First Nations peoples should also lead the design and implementation of their own prevention strategies.30 In terms of other population groups, there was some evidence of differences between the risk factors that contributed less to dementia. For example, obesity (3·7%,) was lower among people of Asian ancestry than the other groups. Moreover, diabetes ranked higher for people of Asian ancestry, and depression lower, than other groups. These differences between groups might reflect cultural factors; however, more research is required to understand these trends and guide the most appropriate interventions.31
Our study has limitations. We defined European and Asian ethnicity based on parental country of birth, as the NHS did not include self-defined ethnicity. In the Australian Census, the ABS uses multiple indicators to determine respondent ethnicity,20 of which parental country of birth is one source of information. The proportion of NHS participants in our study with European (23·4%) or Asian (13·7%) ancestry was lower than the 2021 Census (57·3% and 17·4%, respectively).10
This suggests that we undercounted these two population groups. Although the prevalence estimates were population specific, we used RRs from global meta-analyses. We assumed, as other dementia PAF research has done, that these RRs apply to different populations (ie, First Nations peoples and Australians of Asian and European ancestry). Given that we used these RRs, the PAF estimates reflect risk factors prevalence between groups, and their communalities. Although incidence estimates of traumatic brain injury exist for specific jurisdictions,32 the absence of national data masks the extent to which this condition contributes to dementia nationally and variations between population groups. By omitting traumatic brain injury, our main analyses underestimated the true proportion of preventable dementia.
We only included global risk factors consistently shown to plausibly be causative of dementia in many populations. We did not consider the contribution of risks statistically associated with dementia for First Nations populations, such as childhood trauma.33 Although consistent with the global approach, our selection of risk factors and their RRs might not adequately reflect potentially preventable dementia in First Nations Australians. Future research should broaden investigations to include other potential risk and protective factors specific to First Nations populations.
The ABS data represent Australia’s most comprehensive health indicators, but are mainly self-reported. Participants might under-report factors with negative connotations, such as smoking, alcohol, or hearing loss, leading to underestimation of their prevalence. The ABS survey is adding objective physical measures, and future PAF studies could incorporate these to improve the accuracy of prevalence estimates. The largest dementia risk in this study, physical inactivity, was only recorded for non-remote survey respondents in the NATSIHS. This limits the accuracy of the prevalence estimates underlying the PAF calculations.
Communality calculations required complete cases, so excluded all participants from remote areas in the NATSIHS without physical activity assessment. Like other studies, we assumed that the communality from the subsample could be applied to the broader dataset. We also had to apply the average communalities to all groups’ social isolation, as data were in a different database. Due to small sample sizes, we applied the communalities for all Australians to people of European and Asian ancestry. Small sample sizes also probably contributed to the wide confidence intervals around estimates for different population groups.
Two age-specific prevalence estimates for depression and excessive alcohol consumption for people of Asian ancestry were modified due to small cell sizes. Although supplementary analyses with larger age groups were similar to the main analyses, our study highlights the limitations of using the Australian NHS dataset for small subpopulations. The NHS and NATSIHS did not include prevalence estimates for social isolation, so we sourced this from the GSS and NATSISS and made transparent assumptions of average communality. Traumatic brain injury prevalence data were not available for all Australians or First Nations Australians. Consequently, we used measures from other sources for our supplementary analyses. Finally, our results do not provide conclusive evidence that reducing these risks will produce a decline in dementia in Australia, nor that these findings are generalisable to other populations around the world.
Nonetheless, this study provides comprehensive estimates of potentially modifiable risk factors and dementia in Australia, using reliable health measures. Improving physical activity, preventing and treating obesity, and improving hearing could make a substantial difference to future rates of dementia. There is a particular potential to reduce dementia rates in First Nations Australians and explore differences in specific risk factors between other population groups. These findings add dementia risk reduction evidence about different population groups across Australia to guide future risk reduction programmes and public health policy.
Contributors
The research idea was conceived by FT, RSS, SR, LRH, RQ, ES, ST, AE, and RM. FT obtained approval to access the data and was the primary analyst. RSS and FT accessed and verified the underlying data, led the writing, and collaborated on the analyses. AE and ZH provided statistical advice. RM obtained the funding for this study. GL guided development of the methods. ES, DLG, and KR advised on national implications in the paper. All authors critically reviewed the manuscript, contributed to the content, and approved the final version. RSS had the final responsibility to submit for publication.
Data sharing
Weighted prevalence estimates from the Australian Bureau of Statistics (ABS) surveys used in the current study are available on the ABS website. Aggregated weighted data from these surveys are also hosted by the ABS on their TableBuilder online platform, where users can perform limited analyses (eg, calculating prevalence by age groups). The ABS also stores the deidentified unit record-level data that underly the national surveys on their DataLab online platform. Users can perform more detailed analyses, such as generating variables not available publicly, and all results are vetted by ABS staff before release. Access to ABS TableBuilder and the ABS DataLab requires various approval processes (appendix 1 p 7). The ABS Centre of Aboriginal and Torres Strait Islander Statistics provided approval to access data for First Nations Australians. All results and, where possible, information on underlying raw data used in this manuscript are available in appendix 2. Stata.do files used for data preparation and analyses are available from: https://github.com/FintanJCU/Potentially-modifiable-dementia-Australia.git.
This piece was republished from The Lancet Public Health.